

Our potential first-in-class gene therapy candidate for adrenomyeloneuropathy (AMN) has joined Spur’s expanding pipeline of next-generation genetic medicines. Learn more and get updates at SpurTherapeutics.com.
This site will remain live until Q4 2024 as we transition content to our new site.


We are a fully integrated research and development company pioneering a deep and varied pipeline of gene therapies with an initial focus on the spinal cord.

Our approach leverages the significant progress that has been made in identifying specific, disease-causing mutations.
By manipulating and correcting the “faulty” gene, either:
A) by adding the normal gene
OR
B) by providing new genetic instructions,
we’ll be able to introduce new therapies to help fight disease, changing the way we approach and treat inherited diseases.


Adeno-associated viruses (AAVs) are small, nonenveloped, single-stranded DNA (ssDNA) viruses. Clinical studies have shown AAVs can be promising vectors for delivering therapeutic DNA.
Advances in genetic medicines have validated that AAVs can deliver therapeutic genes directly to the CNS in a well-tolerated and effective manner.
Using AAVs to deliver genetic corrections leverages the promise of gene therapy, replacing damaged genes and treating the root cause of disease. This type of therapy has the potential to provide patients with a durable, disease-altering option, rather than only symptom management, significantly alleviating the burden of disease on patients and caregivers.
We are taking a rational approach to building our pipeline, targeting well-defined disease areas with high unmet medical needs.
Our initial drug development candidate, SBT101, aims to treat adrenomyeloneuropathy (AMN) by targeting the disease-causing mutation in the ABCD1 gene. SBT101 incorporates a novel approach using AAV9, a vector for delivery with demonstrated therapeutic value and a growing body of safety data.
We are currently conducting a Phase 1/2 clinical study of SBT101, along with an ongoing natural history study of AMN.
As our program for AMN advances, our intent is to leverage insights from this program into other spinal cord-related indications to expand our pipeline and aggressively pursue therapies where there is significant need.
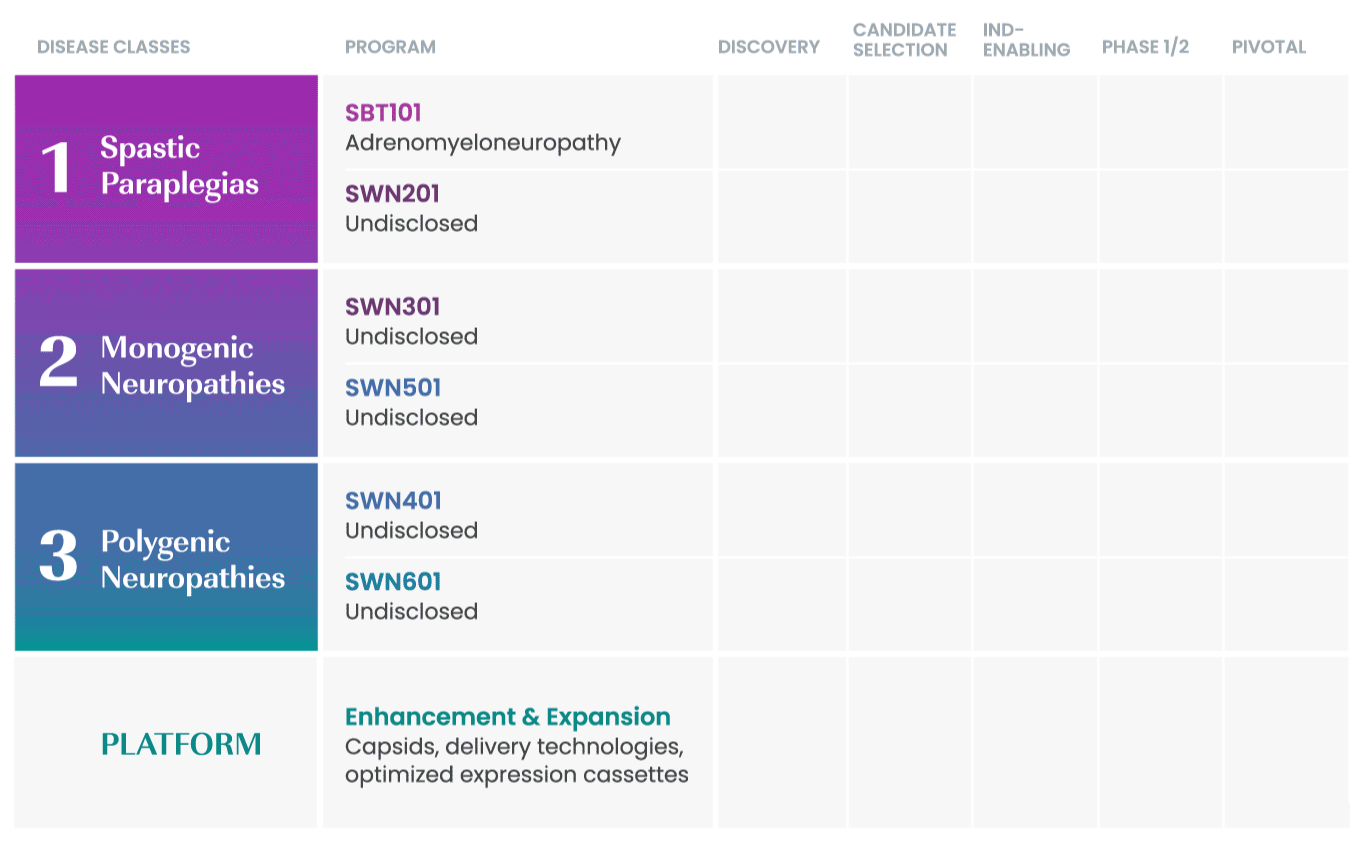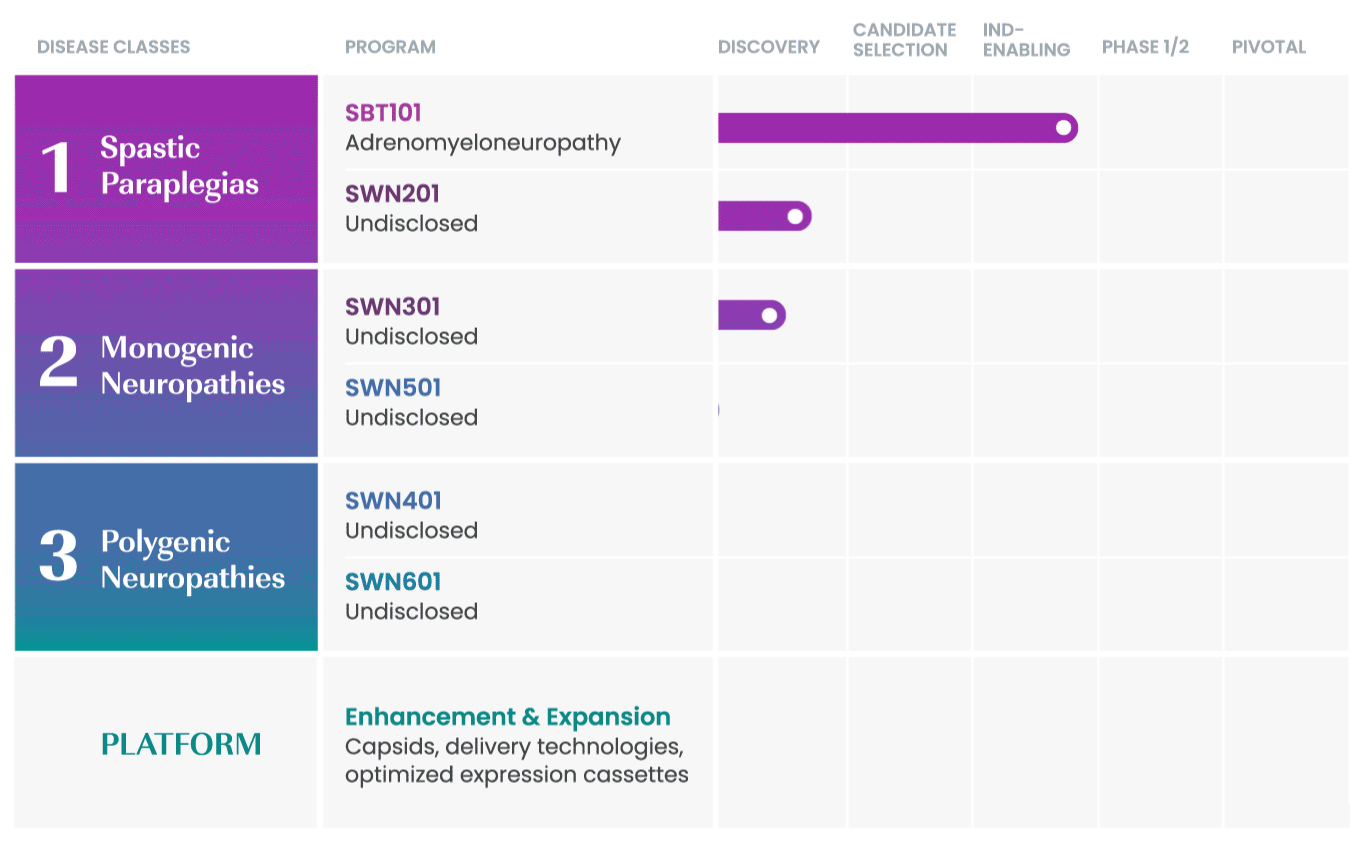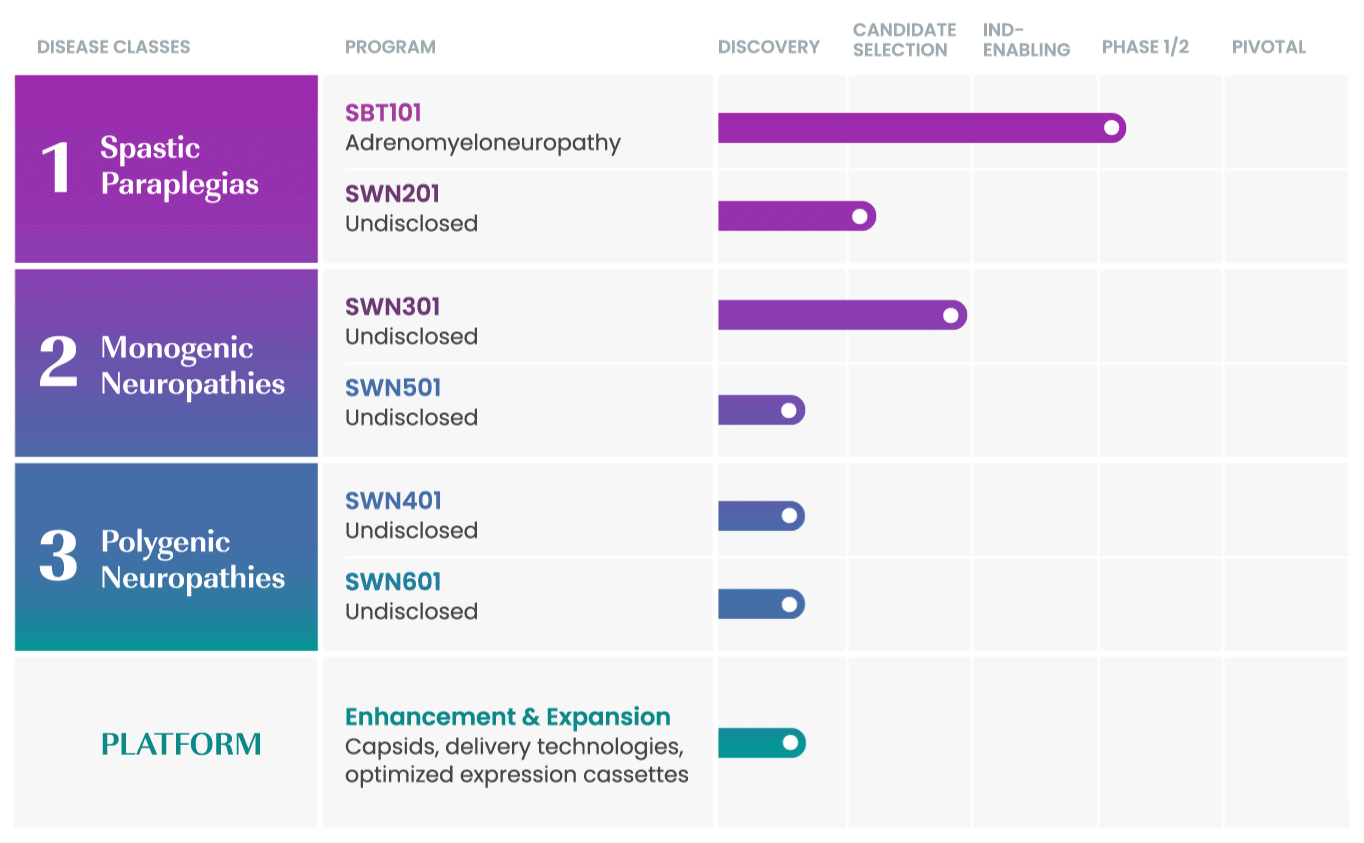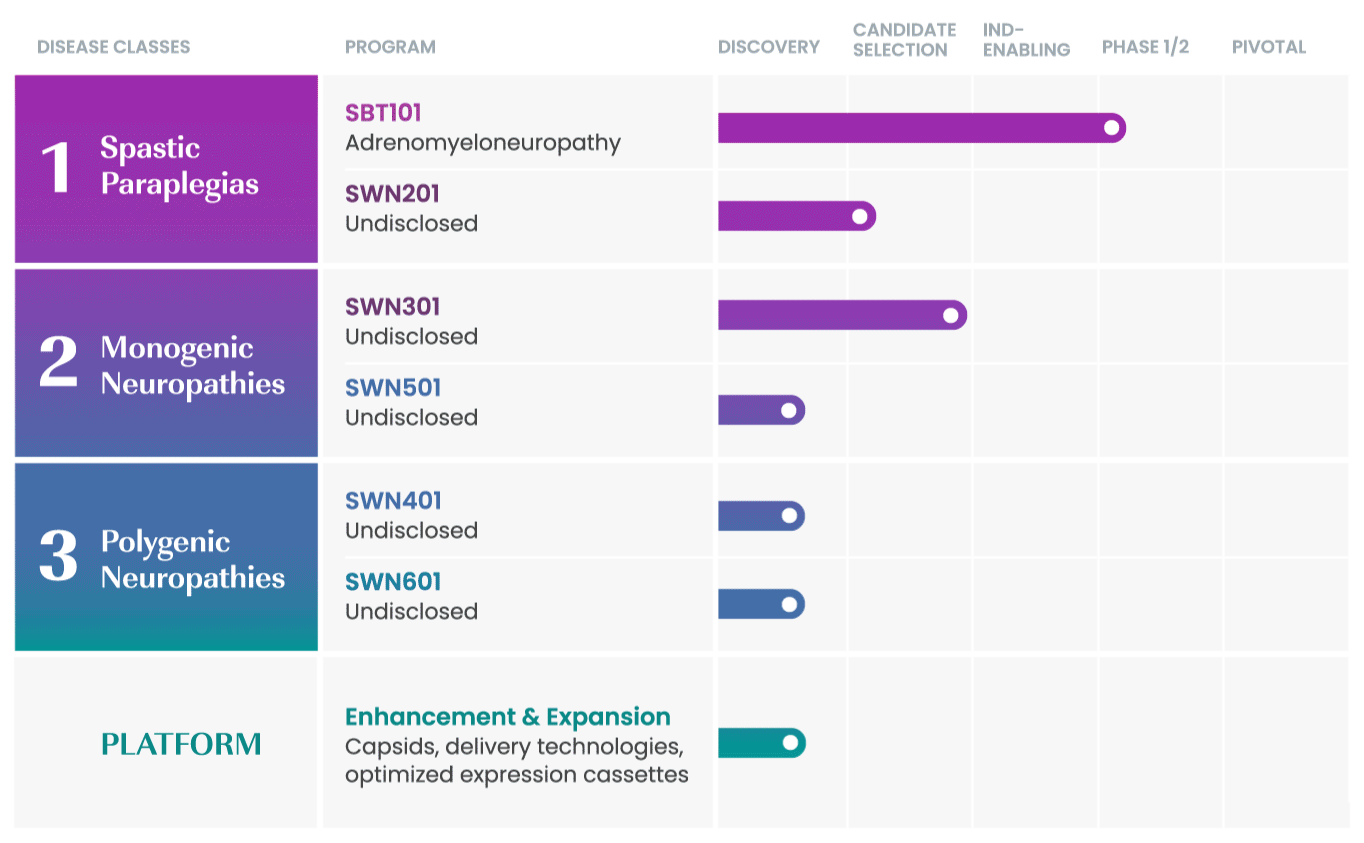
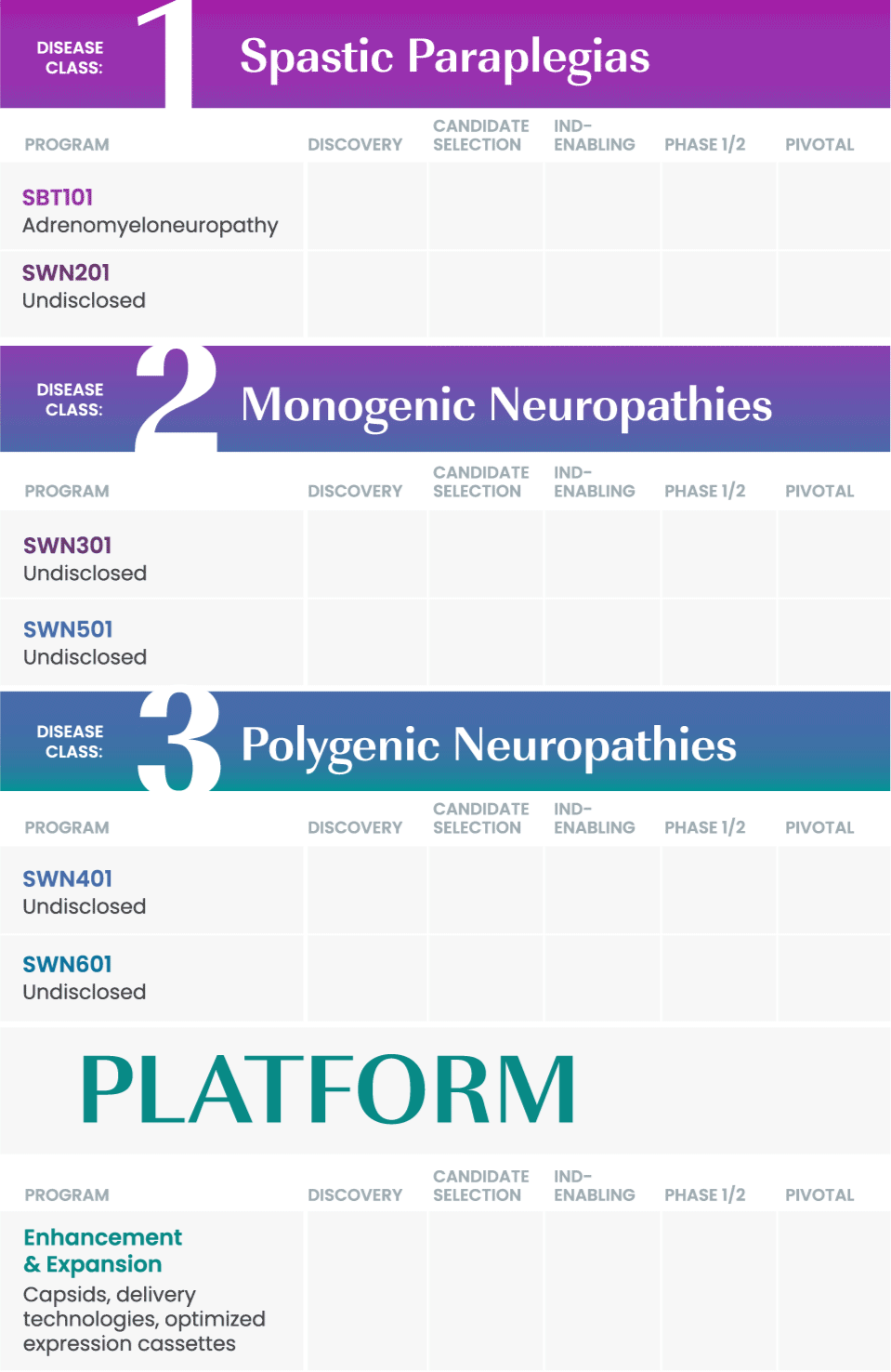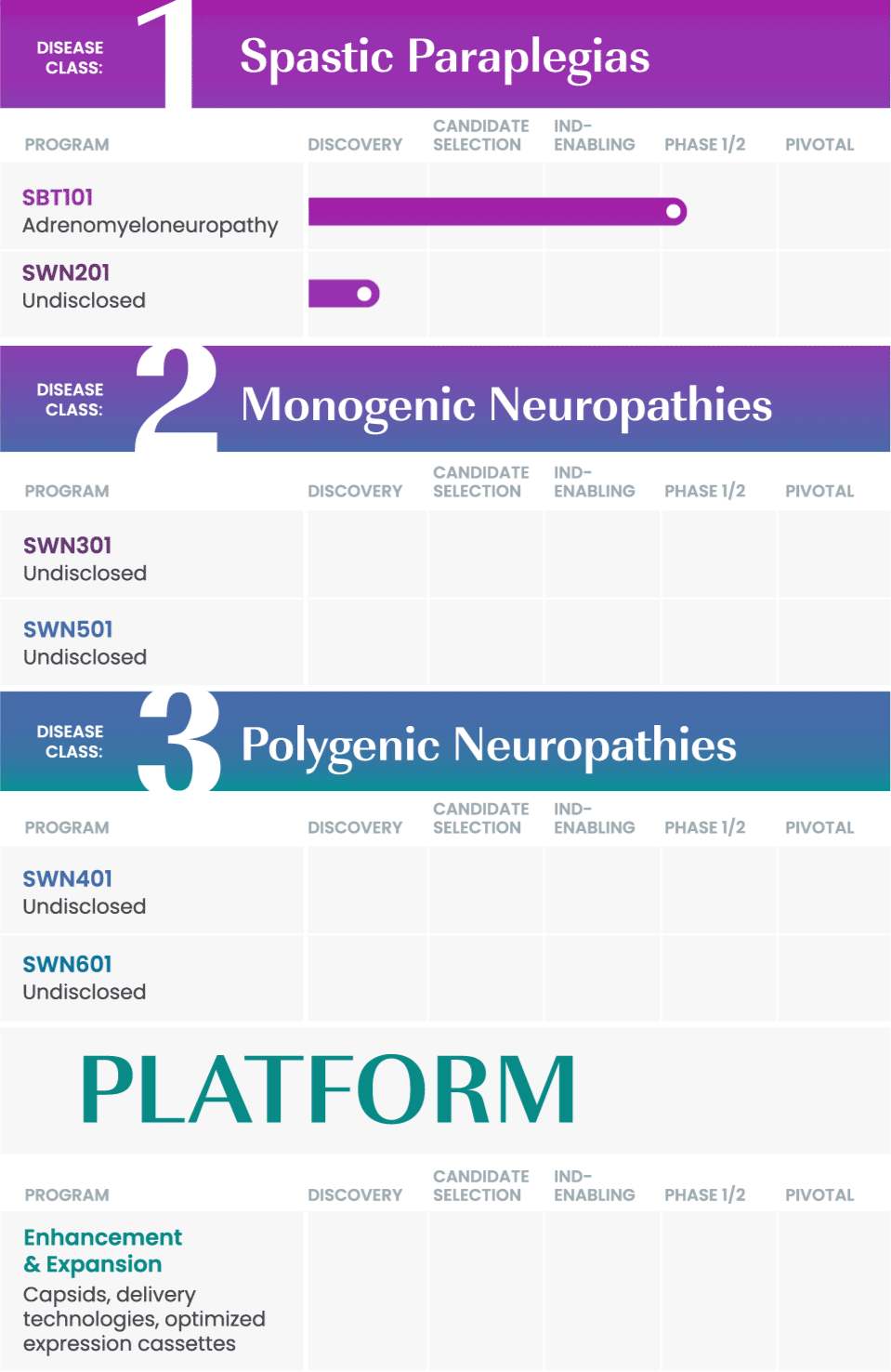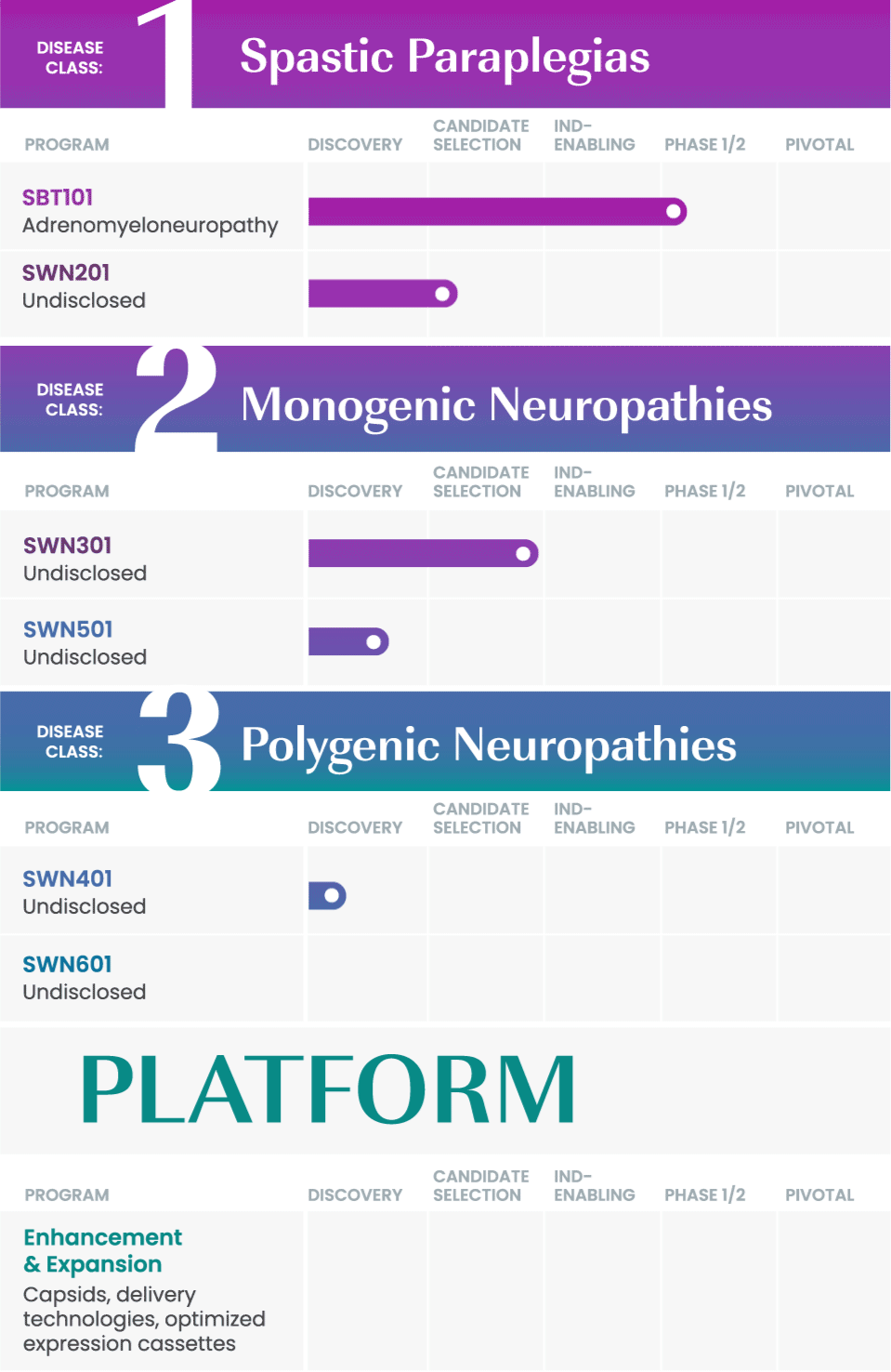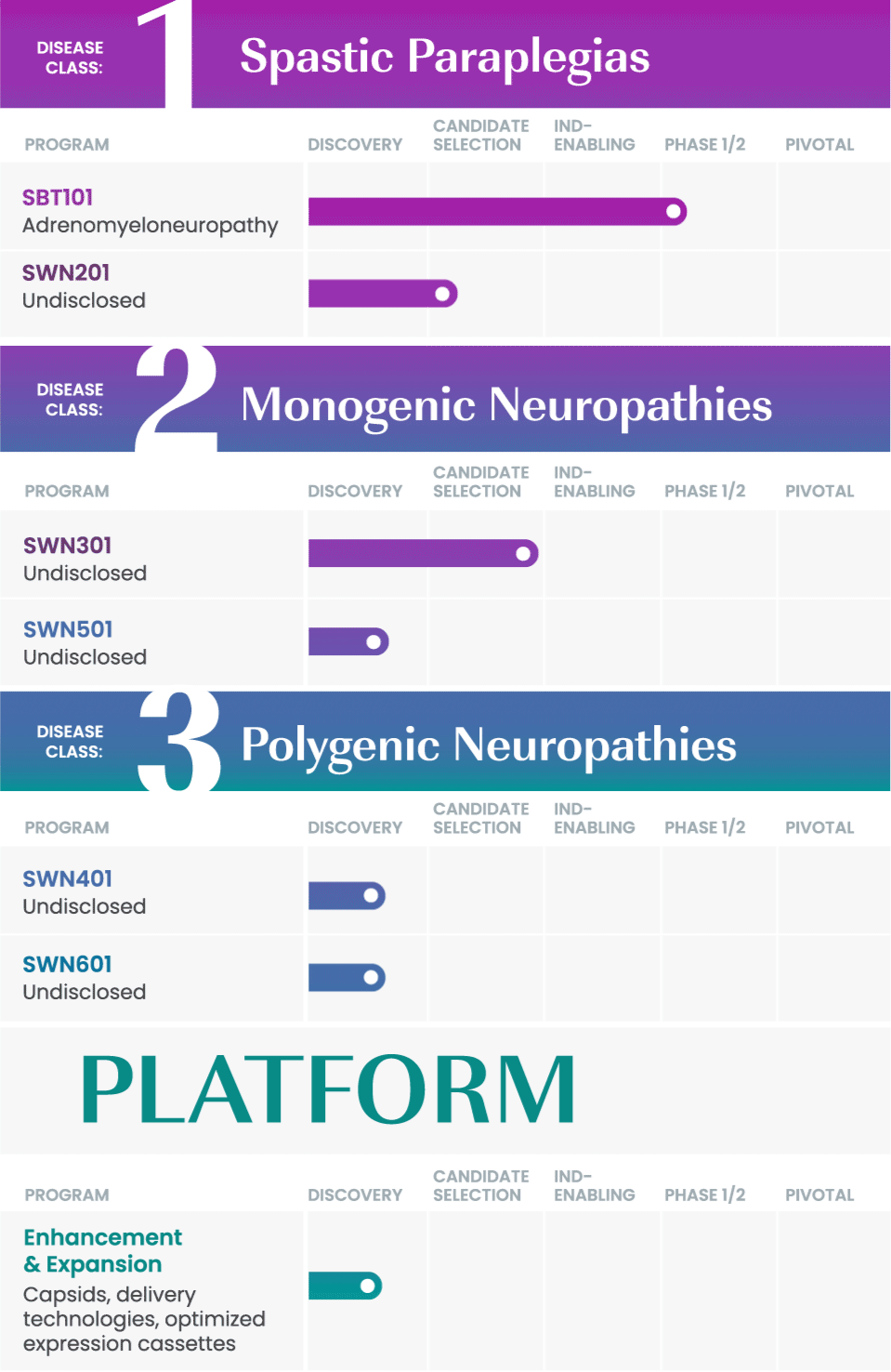
SwanBio collaborates with international key leaders as well as academic institutions and other research organizations to advance the company’s portfolio. For more information about partnering and collaborating with SwanBio please contact our Business Development team at BD@SwanBioTx.com.