

Our potential first-in-class gene therapy candidate for adrenomyeloneuropathy (AMN) has joined Spur’s expanding pipeline of next-generation genetic medicines. Learn more and get updates at SpurTherapeutics.com.
This site will remain live until Q4 2024 as we transition content to our new site.


Inherited neurological disorders are conditions that can be progressive and debilitating, causing problems with balance, walking, sensation (numbness), and other symptoms.
Historically, neurological disorders have presented a unique challenge for drug development. Creating safe and effective drugs for diseases involving the spinal cord requires identifying highly targeted therapies and delivering them to nervous tissues and compartments in the body.
Treatments have been limited and those that are available focus on symptom management, rather than targeting the root cause of the disease.
Our initial focus is on spinal cord-related disorders, beginning with adrenomyeloneuropathy (AMN).

Our vision is to enhance our understanding of the origin and management of spinal cord-related disorders where therapeutic DNA has the potential to treat these debilitating illnesses.


AMN is a progressive and debilitating neurodegenerative disease caused by alterations in the ABCD1 gene. These genetic changes disrupt the function of spinal cord cells and other tissues.
AMN is characterized by loss of mobility in adulthood, incontinence, debilitating pain, and sexual dysfunction, which all affect quality of life.
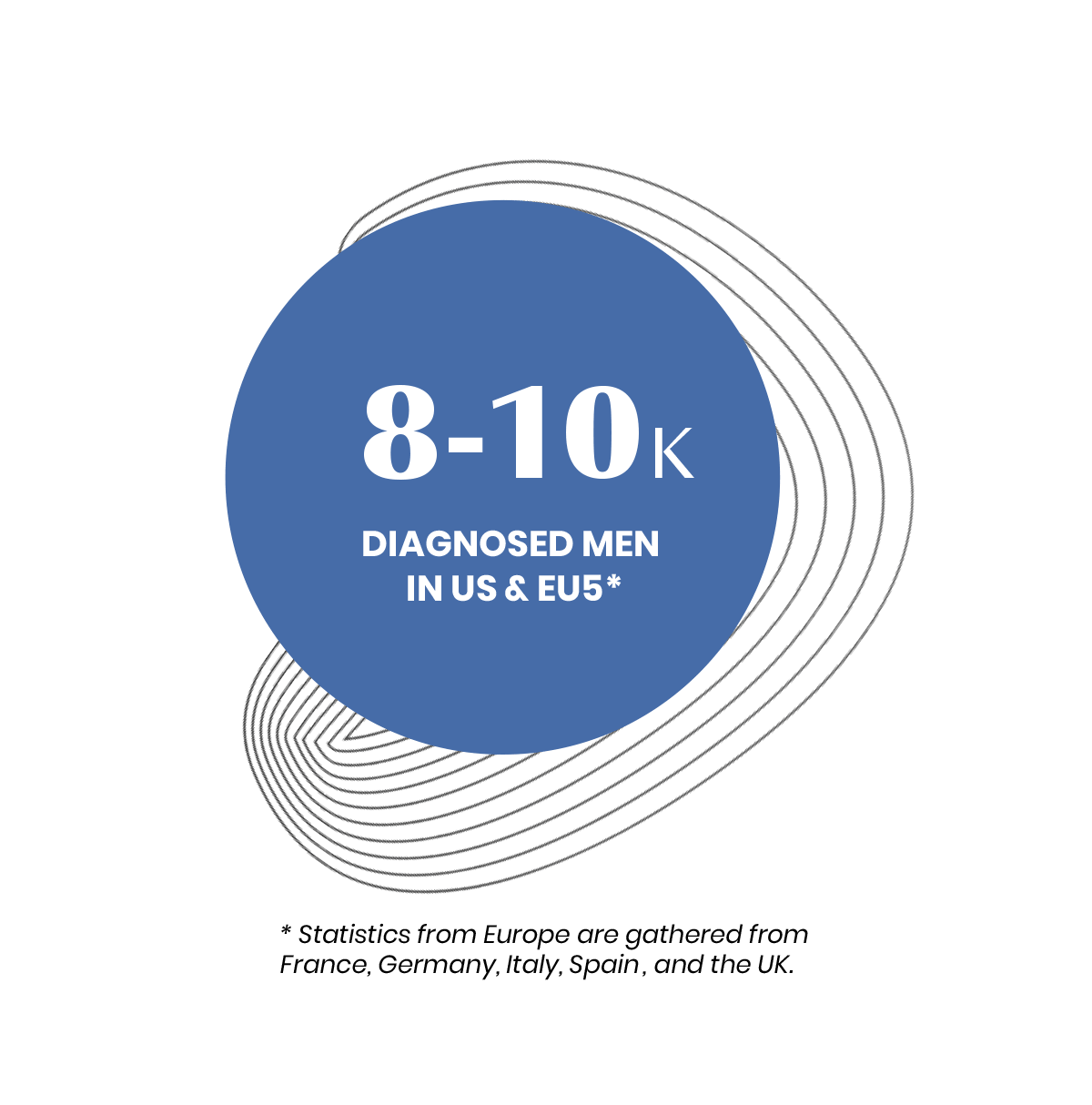
Symptoms of AMN typically begin in men between the ages of 20 and 30. Women can experience symptoms later in life.
There are currently no approved treatments to slow or alter the progression of AMN. Standard of care is symptom management, physical therapy, and mobility aids.


Our approach to treating disease builds on advances in the delivery of gene therapy and targets the root cause of disease.

Through our team’s collective decades of experience, we know that people living with neurological disorders can’t wait.